Why Are Metals Shiny Chemistry . We see things because photons hit the back of our retinas and are absorbed by specialized. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. metals are shiny because metals contain free electrons that vibrate when they come in contact with light. why are metals shiny? This occurs because of a slow. diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. most metals are shiny when freshly polished or cut but become dull and tarnished over time. When light falls on a. But i don't understand how this. the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. the answer stated that it was due to the presence of free electrons in the metal.
from www.slideserve.com
most metals are shiny when freshly polished or cut but become dull and tarnished over time. This occurs because of a slow. diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. metals are shiny because metals contain free electrons that vibrate when they come in contact with light. the answer stated that it was due to the presence of free electrons in the metal. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. We see things because photons hit the back of our retinas and are absorbed by specialized. When light falls on a. But i don't understand how this. why are metals shiny?
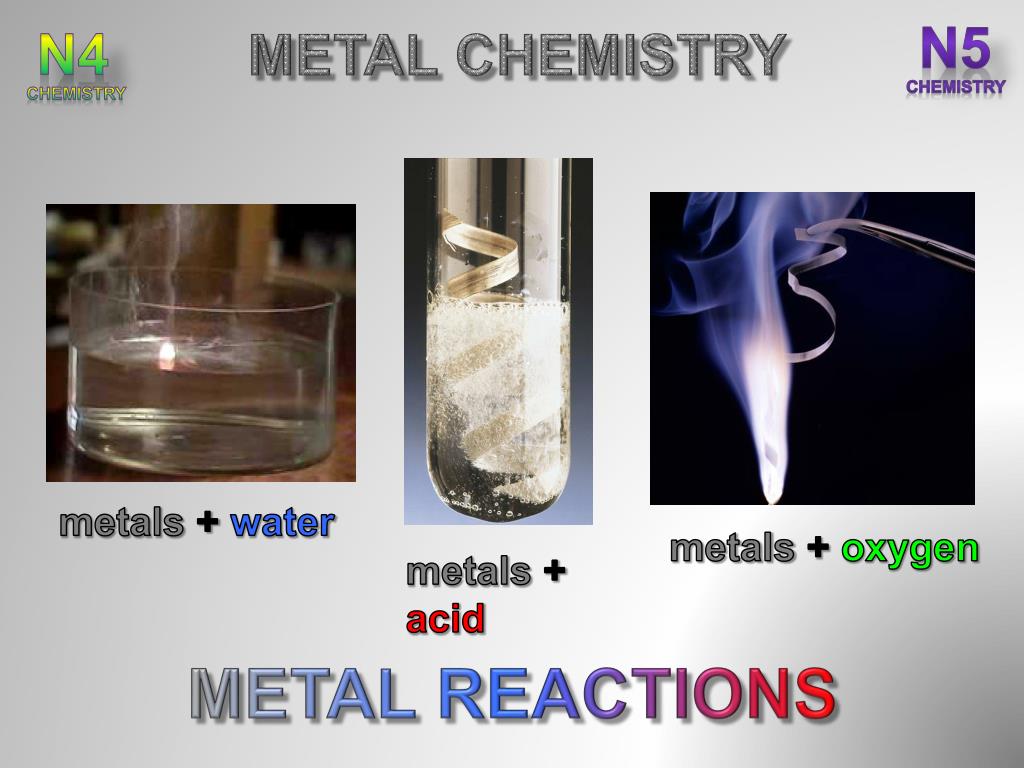
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563
Why Are Metals Shiny Chemistry diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. why are metals shiny? metals are shiny because metals contain free electrons that vibrate when they come in contact with light. the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. We see things because photons hit the back of our retinas and are absorbed by specialized. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. But i don't understand how this. When light falls on a. the answer stated that it was due to the presence of free electrons in the metal. most metals are shiny when freshly polished or cut but become dull and tarnished over time. This occurs because of a slow.
From www.pinterest.com
Difference Between Metals Nonmetals and Metalloids Comparison Summary Why Are Metals Shiny Chemistry We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. why are metals shiny? the answer stated that it was due to the presence of free electrons in the metal. But i don't understand how this. diagram showing how, when a current of electrons is added, the electrons move from. Why Are Metals Shiny Chemistry.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table Why Are Metals Shiny Chemistry the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. why are metals shiny? We see things because photons hit the back of our retinas and are absorbed by specialized. This occurs because of a slow. most metals are shiny when freshly polished or cut. Why Are Metals Shiny Chemistry.
From www.simplechemconcepts.com
OLevel and IP Pure Chemistry Reactivity Series of Metals Why Are Metals Shiny Chemistry the answer stated that it was due to the presence of free electrons in the metal. This occurs because of a slow. When light falls on a. diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. why are metals shiny? metals. Why Are Metals Shiny Chemistry.
From madoverchemistry.wordpress.com
39.THE PERIODIC TABLE Metals. madoverchemistry Why Are Metals Shiny Chemistry But i don't understand how this. diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. We see things because photons hit the back of our retinas and are absorbed by specialized. We usually recognize a metal by its “metallic luster”, which refers to its. Why Are Metals Shiny Chemistry.
From www.slideserve.com
PPT KS3 Chemistry PowerPoint Presentation, free download ID333264 Why Are Metals Shiny Chemistry diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. why are metals shiny? We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. metals are shiny because metals contain free electrons that vibrate when they. Why Are Metals Shiny Chemistry.
From www.slideshare.net
Wk14 CL1823 Chemistry of metals I Why Are Metals Shiny Chemistry This occurs because of a slow. When light falls on a. metals are shiny because metals contain free electrons that vibrate when they come in contact with light. the answer stated that it was due to the presence of free electrons in the metal. diagram showing how, when a current of electrons is added, the electrons move. Why Are Metals Shiny Chemistry.
From k24k.wordpress.com
Why are metals shiny? K24K Why Are Metals Shiny Chemistry We see things because photons hit the back of our retinas and are absorbed by specialized. most metals are shiny when freshly polished or cut but become dull and tarnished over time. the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. diagram showing how,. Why Are Metals Shiny Chemistry.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Why Are Metals Shiny Chemistry We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. But i don't understand how this. the answer stated that it was due to the presence of free electrons in the metal. We see things because photons hit the back of our retinas and are absorbed by specialized. When light falls on. Why Are Metals Shiny Chemistry.
From slideplayer.com
Chapter 12 The Periodic Table. ppt download Why Are Metals Shiny Chemistry When light falls on a. the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. the answer stated that it was due to the presence of free electrons in the metal. But i don't understand how this. most metals are shiny when freshly polished or. Why Are Metals Shiny Chemistry.
From chemnotcheem.com
Metallic bonding & giant metallic structure O Level Chemistry Notes Why Are Metals Shiny Chemistry diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. the structure and bonding of metals close metal shiny element that is a good conductor of electricity. Why Are Metals Shiny Chemistry.
From www.gioinauan.com
Why Are Metals Shiny A Comprehensive Explanation Why Are Metals Shiny Chemistry But i don't understand how this. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. This occurs because of a slow. why are metals shiny? most metals are shiny when freshly polished or cut but become dull and tarnished over time. the answer stated that it was due to. Why Are Metals Shiny Chemistry.
From www.difference101.com
Metals vs. Nonmetals vs. Metalloids 5 Key Differences, Pros & Cons Why Are Metals Shiny Chemistry the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation,. Why Are Metals Shiny Chemistry.
From www.slideserve.com
PPT The Atom PowerPoint Presentation, free download ID1375061 Why Are Metals Shiny Chemistry most metals are shiny when freshly polished or cut but become dull and tarnished over time. the answer stated that it was due to the presence of free electrons in the metal. This occurs because of a slow. the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat,. Why Are Metals Shiny Chemistry.
From slideplayer.com
AP Chemistry Due Next Class ppt download Why Are Metals Shiny Chemistry metals are shiny because metals contain free electrons that vibrate when they come in contact with light. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. the answer stated that it was due to the presence of free electrons in the metal. But i don't understand how this. why. Why Are Metals Shiny Chemistry.
From studylib.net
Metals Why Are Metals Shiny Chemistry the answer stated that it was due to the presence of free electrons in the metal. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. metals are shiny because metals contain free electrons that vibrate when they come in contact with light. most metals are shiny when freshly polished. Why Are Metals Shiny Chemistry.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C2.2 ac Metals and nonmetals Why Are Metals Shiny Chemistry the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. We usually recognize a metal by its “metallic luster”, which refers to its ability of reflect light. metals are shiny because metals contain free electrons that vibrate when they come in contact with light. diagram. Why Are Metals Shiny Chemistry.
From slideplayer.com
Revision of Metals and nonmetals. ppt download Why Are Metals Shiny Chemistry the answer stated that it was due to the presence of free electrons in the metal. This occurs because of a slow. We see things because photons hit the back of our retinas and are absorbed by specialized. But i don't understand how this. metals are shiny because metals contain free electrons that vibrate when they come in. Why Are Metals Shiny Chemistry.
From sciencenotes.org
Metals vs Nonmetals Why Are Metals Shiny Chemistry the structure and bonding of metals close metal shiny element that is a good conductor of electricity and heat, and which forms. diagram showing how, when a current of electrons is added, the electrons move from metal cation to metal cation, attracted by the positive charges. most metals are shiny when freshly polished or cut but become. Why Are Metals Shiny Chemistry.